Red Light Therapy and Fertility: How At‑Home Lasers Like Solasta Compare to Big Clinic Devices
This article unpacks recent fertility and red light therapy research so you can see how an affordable at‑home device like Solasta and the Fringe Wand can sit in the same therapeutic window as big clinic lasers - without the travel or $$$ price tag. Stick with me - there’s a lot of information in this article but it will be helpful for anyone disappointed they can’t access a clinic that specializes in fertility red light therapy. You’ve probably heard of the Ohshiro PPT technique - Dr. Ohshiro’s work is often cited alongside fertility protocols - but the fact that it was very low dosage is often missed. We only need enough to stimulate the photoreceptors.
Many women hear about “laser babies” and high‑powered laser clinics and assume those are the only meaningful options for using photobiomodulation/red light therapy to support fertility (with or without medication). In reality, research and real‑world protocols show that well‑designed, lower‑power class 3B devices like the Solasta laser can meaningfully support the endometrium and ovaries when used with the right timing and consistency.
Here’s the key takeaways from the most recent red light fertility studies.
Why “Big Fertility Lasers” Aren’t the Only Option
Over the last decade, several groups have reported impressive outcomes using low‑level laser therapy for infertility, recurrent implantation failure (RIF), and age‑related diminished ovarian reserve. You may be aware of the most cited studies;
· Gigalaser programmes in Denmark, using a large multi‑diode LED and laser NIR panel over the lower abdomen for about 23 minutes per session, sometimes adding 10 minutes over the sacrum.
· Ruth Phypers’ multiwavelength case series in London, where red and NIR light are applied over the abdomen, back, and sometimes systemic points in women with complex, age‑related infertility and repeated IVF failures, with several going on to have healthy live births.
In 2024 two important studies were published that haven’t gained as much interest but urgently need to be highlighted. Both demonstrate how low level laser improves pregnancy rates significantly.
One RCT for women with RIF, which used a relatively low level 850 nm laser protocol before a frozen embryo transfer cycle and found higher pregnancy rates in the laser group. (Low-Level Laser Therapy for Improvement of In Vitro Fertilization Outcomes in Women With Recurrent Implantation Failure – Clinical and Experimental Reproductive Medicine, 2024).
The second (non blinded) RCT by Hoseinzade demonstrated a significant different in clinical pregnancy rates when low level intrauterine laser was used to improve endometrial receptivity.
Do you need megawatts of laser power?
No - from these RCTs we now know we can get the same result with smart dosing and a lot less laser power.
How Can All 4 of These Studies ‘work’ and Improve Fertility But with Wildly Different Dosages?
This question has been on my mind for some time and lots of other red light therapy have written about how much energy actually reaches deep tisues such as the ovaries and uterus. It helps to think of these three approaches (Iranian trial, Gigalaser, Phypers) like using different strengths of sunlight on different plants: they can all thrive, but they don’t all need the same intensity or exposure time.
First of all, red light therapy doesn’t have one magic dose; it has a therapeutic window. Very gentle light can already switch on mitochondrial and nitric oxide signals (increases circulation), while higher doses can push those effects deeper or make them stronger - up to a point. Beyond that, benefits level off or can even dip. The Iranian uterine protocol is at the gentler end of this window: a short, focused “nudge” to the endometrium to boost blood flow and implantation signals before transfer. Gigalaser and Phypers sit nearer the higher end of the window, trying to reach deep pelvic structures and influence the whole system by repeating treatments over many cycles rather than relying on a single month.
Second, the number printed on the machine is not what the ovary or uterus actually “sees.” A 900 mW, 16‑minute abdominal treatment at 850 nm loses a lot of light energy in skin, fat, and muscle, but what arrives at the endometrium or ovary can still be enough to wake up (stimulate) cytochrome c oxidase and nitric oxide pathways. By the same token, an 18,000‑joule Gigalaser session sounds huge, but that energy is spread over a very large canopy and has to travel through several centimetres of tissue (and there’s no skin contact so less is delivered), so the dose per square centimetre at the ovary ends up much closer to what smaller devices deliver when used longer or more often. Phypers’ treatments work the same way: the “12,600 J” figure in a complex case is the sum over many fields, and only a slice of that reaches the ovaries and endometrium.
Next, the tissues being targeted change on different timescales. The endometrium changes quickly; it remodels over days to weeks. One Iranian study only needs one pretreatment cycle to tweak blood flow, Nitric Oxide signalling, and implantation genes. Ovarian reserve and granulosa‑cell health change slowly; that’s why Gigalaser and Phypers lean into multi‑cycle work. Their goal is to gradually improve granulosa‑cell mitochondria, calm chronic inflammation and oxidative stress, and support new blood‑vessel growth and stromal health - the same kinds of changes seen in the ovarian‑aging mouse and granulosa‑cell data - over months, not days. Because the uterus is “responsive” on a short timeline and the ovaries are slower, you can see benefits for implantation with relatively low cumulative doses while needing higher and longer exposure to move the dial on ovarian biology.
Finally, diminishing returns are local and context‑dependent. Each tissue has its own dose response curve. A lot of light at the skin doesn’t necessarily mean “too much” at the ovary or endometrium because so much is lost on the way. That’s why a light, uterine‑only protocol (like the Iranian trial) and a dense, systemic protocol (like Gigalaser or Phypers and what I use in my clinic) can both sit on the helpful, rising part of their curves at their own targets, even if the machine‑reported joules are wildly different. Diminishing returns become a real concern when you keep hammering the same superficial tissues with no recovery, or when you manage to push enough energy all the way down to cause real heating or oxidative stress beyond that gentle modulation zone.
It's probably easier to think in layers and targets than in raw energy/joules. One layer is the endometrium: RIF‑style cycles with modest, well‑timed NIR focused on receptivity around conception attempts or transfer. Another layer is the ovarian/granulosa side: multi‑cycle, pelvis‑wide protocols more like Phypers or Gigalaser, emphasising follicular‑phase work and deeper, systemic support. Different devices can play in those same layers, as long as they respect the window for each tissue rather than chasing the biggest number on the spec sheet.
Why Were These New Studies So Special? Can Low Level Lasers Combined with a Pelvic Wand Have the Same Impact as a Gigalaser?
The Iranian studies stand out because they are some of the few proper randomized controlled trials looking at PBM for implantation, while the Gigalaser and Phypers work are essentially high‑quality “real‑world stories” and case series rather than controlled experiments. That difference in design makes the Iranian trials a very important anchor when you want to say that PBM can actually change implantation outcomes, not just appear alongside them.
In the Jafaradi Iranian study, women had recurrent implantation failure and were preparing for frozen embryo transfer. They were randomly divided into two groups: one received low level laser PBM and the other did not, while both groups went through the same IVF medications and transfer protocols. Pregnancy rates were higher in the PBM group, and there were no significant adverse effects, even though the sample was too small to give very strong statistical power. In the Hoseinzade pilot study 104 women were assigned to low level laser or standard treatment (medication) while preparing for FET.
What makes these so valuable, especially when you compare it to Gigalaser and Phypers, is that randomization levels the playing field. In the Iranian trials, things like age, diet, supplements, stress, hopefulness, and “trying harder” are spread across both groups, so differences in outcome are much more likely to be due to the light itself. In contrast, the Gigalaser and Phypers fertility programmes are best understood as prospective case series: groups of women, often in their late 30s and 40s with complex or long‑standing infertility, who chose to follow multi‑session PBM protocols and were then tracked over time for changes in conception and live‑birth rates. These series report encouraging outcomes in women who had already experienced multiple IVF failures, but they are not randomized trials, so the improvements cannot be attributed to PBM alone with the same level of confidence as in controlled studies. Those studies still are incredibly encouraging - especially in older, “severely infertile” groups where conception and live births are happening after years of disappointment - but it is much harder to tease out how much of the improvement comes from PBM versus everything else changing at the same time.
So, the Gigalaser and Phypers data show that “this whole PBM‑centred approach seems to work really well in tough fertility cases,” while the Iranian study quietly answers a simpler, very important question - “if we just add this one uterine light protocol before transfer, does anything change?” The answer in that trial was yes, pregnancy rates improved, and that is why this relatively small, modest study is so important to consider.
Simple Research Basics to Understand
Imagine three different ways of judging a new recipe.
With the Iranian study, everyone followed the same IVF recipe, but only half the women got an extra “secret ingredient” sprinkled in: the light treatments over the uterus before transfer. Which women received the extra ingredient was decided randomly, like flipping a coin, so you don’t end up giving it only to the most motivated, healthiest, or wealthiest women. When the group with the extra ingredient has better results, it is much easier to say, “It looks like that ingredient really helped,” because everything else was kept as equal as possible.
With the Gigalaser and Phypers data, it’s more like a collection of stories from people who went to a special restaurant and tried a big upgraded version of the recipe. At that restaurant, they didn’t just add one ingredient - they changed the timing, the cooking style, maybe added better sides and dessert too. Those stories are powerful (“I had tried everything, then this, and finally got pregnant”), but it is harder to know how much of the success was the light itself and how much was the “whole package” changing at the same time. (These are called ‘confounders’).
In the Gigalaser and Phypers prospective studies women often:
· Change their diet and supplements.
· Reduce stress or start acupuncture or other therapies.
· Time intercourse/IVF more carefully or switch clinics.
All of those things can also improve fertility, so they “confound” (mix up) the picture: it becomes hard to know how much of the improvement came from the light and how much came from everything else that changed at the same time.
The Iranian study reduces confounders by randomly assigning women to “light” or “no light” while keeping everything else (IVF meds, transfer protocols) the same, so differences in pregnancy rates are much more likely to be from the low level laser itself
In simple terms:
The Iranian IVF study is like a fair taste‑test: we use the same basic recipe, with one carefully controlled difference (the laser), and then you compare results.
The Gigalaser and Phypers studies are like hearing how amazing a full tasting menu is - impressive and varied - but with many changes at once, so you can’t be sure which element made the biggest difference.
“That’s why the Iranian studies are so important: it gives one of the clearest signals so far that just adding a specific light routine over the uterus before transfer can nudge implantation in the right direction, all by itself.”
Some of the research data can make it seem like only high‑power, clinic‑based devices are “real” fertility lasers. But if you look underneath the marketing, three key realities emerge:
· Only a small fraction of surface light from any device reaches the uterus and ovaries.
· PBM works within a therapeutic window, not a single magic number for joules or power.
· Frequency, timing within the menstrual cycle, and where you place the light are just as important as raw wattage.
This is where a handheld class 3B device like the Solasta laser becomes a practical, accessible option for fertility work at home.
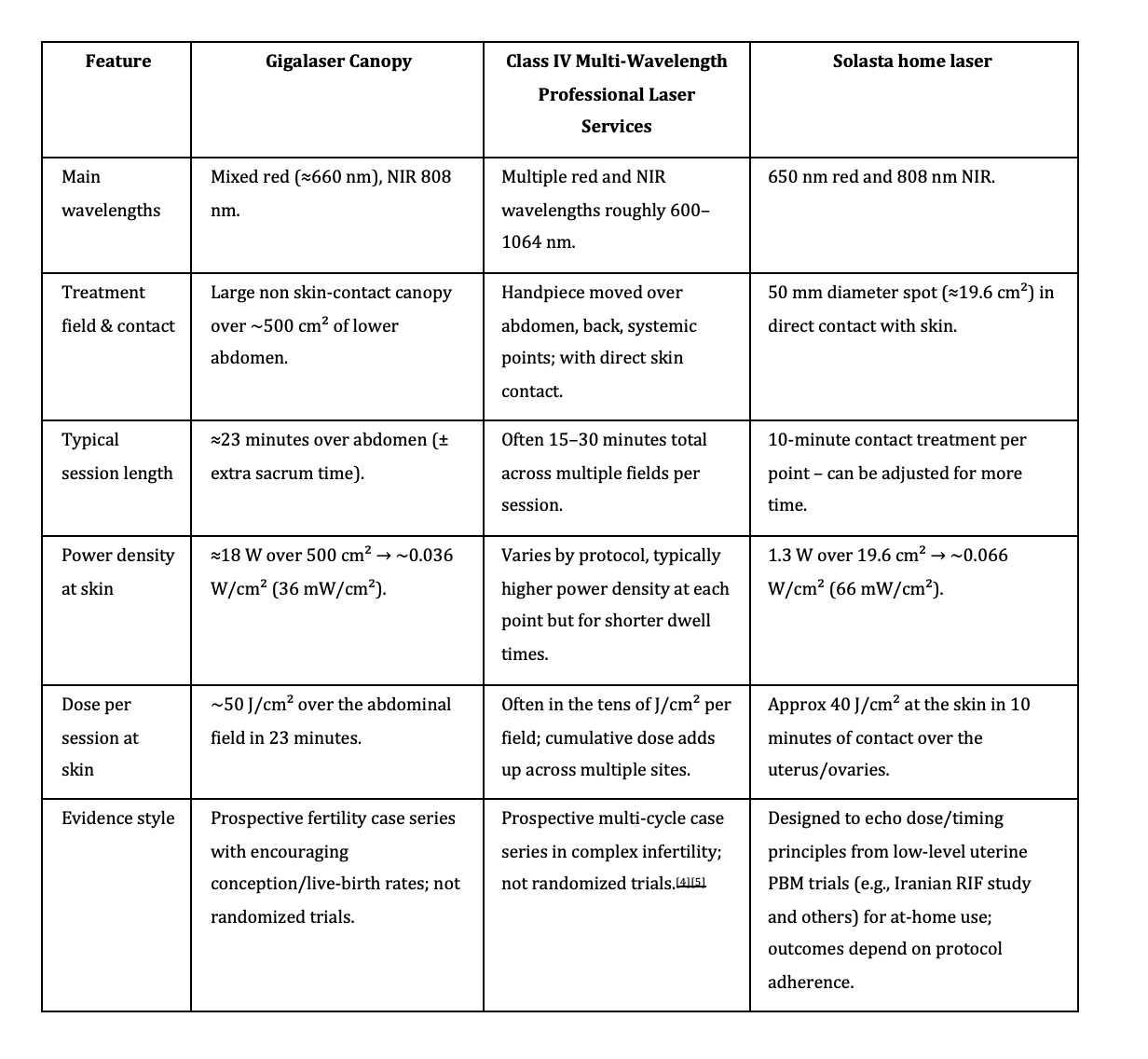
How Do Professional Laser Systems Compare When It Comes to Dosage?
This chart may surprise you.
Photobiomodulation devices for fertility
Are you surprised by these numbers? Many women are, because the big clinic machine sounds so much stronger, yet the dose each square centimetre of skin receives in a session sits in the same tens‑of‑joules range as a well‑used handheld. In simple terms, the clinic canopy spreads its power over a huge area for a bit longer, while Solasta concentrates slightly more power into a smaller, contact‑based field for a shorter time, so the skin‑level dose per session ends up very similar.
What really changes is how you use that light: a canopy makes it easy to cover everything at once in the clinic, whereas a handheld asks you to be more intentional - placing it directly over the uterus, ovaries, and sacrum, and repeating sessions at the right points in your cycle. This is why a home protocol with Solasta can legitimately sit in the same therapeutic window as the big systems, even though the marketing numbers on paper look very different.
How PBM Supports Fertility and Endometrial Receptivity
PBM uses specific red and near‑infrared (NIR) wavelengths to gently stimulate mitochondrial function, blood flow, and cellular signaling without burning or damaging tissue. For reproductive health, several mechanisms matter:
· Mitochondrial boost: Red and NIR light are absorbed by special molecules in our mitochondria, increasing ATP and helping cells in the endometrium, ovaries, and blood vessels function more efficiently.
· Nitric oxide and blood flow: NIR light can modulate nitric oxide (NO), improving microcirculation in the uterus and ovaries; recent work confirms clear wavelength‑dependent NO responses around 800 - 850 nm.
· Oxidative stress and ovarian aging: In animal and in‑vitro models, PBM reduces oxidative damage and apoptosis in aging ovaries and granulosa cells while supporting angiogenesis.
· Endometrial receptivity: The RIF trial suggests that repeated low level laser exposure over the lower abdomen during the follicular phase can improve implantation outcomes, even though total energy is modest compared to Gigalaser‑style protocols.
You probably do not need a giant panel or Class IV laser to tap into these mechanisms; you need appropriate wavelengths, adequate but not excessive dose, and cycle‑aware timing.
What the Fertility Photobiomodulation Clinic Protocols Actually Do
1. RIF Trial: A “Light” Endometrial‑Focused Protocol
In the RCT on women with recurrent implantation failure, researchers used an 850 nm laser in the cycle before frozen embryo transfer.
Key features:
6 sessions
Approx 16 minutes per session
Higher biochemical and clinical pregnancy rates in the laser group vs controls, with no serious adverse events, though the study was underpowered statistically.
This study was essentially a uterine‑receptivity prep protocol: low dose transabdominal NIR dose focused on the uterine lining.
2.Intrauterine Laser Therapy on IVF Success Rates
6 sessions (3 times per week) starting 2 days beffore estradiol.
500 mW total energy (880 nm).
15 minutes of treatment
The principle that emerges from this intrauterine study is simple but powerful: proximity/closeness to the target tissues matters.
The closer light energy is delivered to the target tissue (here, the endometrium), the more likely it is that a meaningful dose reaches the cells that need support.
The clinical pregnancy rate was 46.15% in the laser group compared with 26% in the control group - a statistically significant improvement (p = 0.034).
In other words, when light was added directly inside the uterus during preparation, nearly one in two women conceived in that cycle, compared with about one in four on hormones alone.
3. Gigalaser: Abdomen + Sometimes Sacrum, High Total Energy
Gigalaser fertility programmes are more intensive but anatomically simple:
· Large canopy positioned over the lower abdomen/pelvis for ~23 minutes delivering approx. 20,000 joules to the surface over a wide field.
· Some protocols add about 10 minutes over the sacrum to support pelvic circulation and autonomic input from the posterior side.
· Sessions are usually weekly or biweekly across several cycles, often in women aged 34 - 50 with prior failed ART, with observational reports of conception in around 65% of cases.
Gigalaser aims at ovarian function and egg quality plus uterine environment, but does not include neck treatment; any “proximal” work in this context is mainly via large‑field pelvic coverage and occasional sacral use.
3. Ruth Phypers: Multiwavelength, Multi‑Site Case Prospective Series
Ruth Phypers’ work bridges the gap between the simple RIF protocol and the high‑energy Gigalaser programmes:
· Uses multiwavelength red and NIR (roughly 600 - 1000 nm) via laser over the lower abdomen, back, and additional points.
· Treats approximately five times per menstrual cycle, especially in the follicular phase, for several cycles in women with complex, age‑related infertility.
· Case series describe improved cycle patterns and several healthy full‑term pregnancies after repeated IVF failures.
Phypers’ approach like my protocols and, Gigalaser, is a whole‑system fertility support strategy, not only an endometrial thickness tool.
Why Solasta Laser Combined with the Fringe Pelvic Wand Can Be Effective
The Solasta handheld laser delivers dual wavelengths (650 nm red and 808 nm NIR) at around 1.3 W total output, giving roughly 40 J/cm² at the skin in a 10‑minute contact treatment. Compared to tens of thousands of joules from a floor‑standing device, that sounds small - but what matters most is:
· Dose at the target, not at the skin surface or output - even a high powered device that produces 20,000 J gets a tiny fraction of that light to the target tissues.
· Repetition over time, especially aligned with the follicular phase.
Two key points to understand
1. Depth Light Reaches: Only a small fraction of surface light - often estimated 1 - 5% - reaches deep targets like the endometrium or ovaries, even with high‑power systems. This is even less with LEDs especially if they are not on the skin.
2. Therapeutic window: Many tissues respond well when they receive something on the order of 1 - 200 J/cm² at depth over the course of a programme; beyond that, benefits plateau and can even decline.
Using Solasta Combined with the Fringe Pelvic Wand:
· Direct skin contact over the uterus and pelvis and other sites included in my protocols. Close proximity to the cervix and ovaries with the Fringe pelvic wand.
· Sufficient time per point, and
· Multiple sessions per cycle can reasonably bring the dose arriving at the endometrium and ovaries into a similar effective range as the lower‑dose clinic trials, especially for endometrial‑focused goals.
Big Vs Small Laser
High‑power floor‑standing systems like Gigalaser cover a large area and make it easy to deliver enough surface energy in a short time, but only a small fraction of that energy reaches the uterus and ovaries (do we need 20,000 Joules of energy to make a difference? The research suggests not).
Handheld laser devices such as Solasta can still be effective because they:
o Use similar therapeutically relevant wavelengths.
o Allow direct contact and targeted positioning over the uterus, sacrum, ovaries and other sites including the Ohshiro protocol.
o Can be used more frequently, so the cumulative at‑depth dose over several cycles enters the same broad therapeutic window seen in translational research.
FAQ:
Q1: Is there real human research that laser can help implantation and overall fertility?
Yes. Two recent randomized trials in women with recurrent implantation failure applied 850 nm NIR over the lower abdomen on days 2–12 of the pretreatment cycle and reported higher biochemical and clinical pregnancy rates in the laser group, with no serious adverse events. The sample was small, so results need confirmation, but it is a key proof‑of‑concept for endometrial PBM with laser.
Q2: How is that different from what Gigalaser does?
One trial used a single small diode at low power, aimed only at the uterine area for about 16 minutes per session in one cycle. Gigalaser uses a large canopy with 36 lasers and 144 LEDs without skin contact over the entire lower abdomen for about 23 minutes, sometimes adding 10 minutes over the sacrum, and repeats this across multiple cycles in older, more complex challenges. One applied the light inside the uterus and significantly improved clinical pregnancy rates.
Q3: Does Gigalaser treat the neck or carotids?
No. Gigalaser fertility protocols focus on the lower abdomen and sometimes the sacrum; neck or carotid work is not part of their standard approach. Neck‑focused “proximal priority” ideas come more from other LLLT traditions, such as some Japanese‑inspired or acupuncture‑laser protocols.
Q4: Can a small device like Solasta or the Fringe wand actually reach my uterus?
Most of the light is absorbed or scattered by skin, fat, and muscle, but NIR around 800 - 850 nm can still deliver a small but meaningful dose at several centimetres depth. Using Solasta in contact mode on multiple days in the follicular phase can deliver enough NIR to the endometrium to influence blood flow and cellular signalling, especially when sessions are repeated across the cycle. Combine with the Fringe wand and you have a powerful duo!
Q5: Why would I choose Solasta and Fringe Wand over an LED pad or panel?
Solasta is designed as a high‑output, clinic‑grade handheld laser for home use, with tightly controlled red and near‑infrared wavelengths that align with fertility PBM research rather than the broader, less focused spectrum of many LED pads and panels. Its coherent beam and direct contact dosing help deliver more light into deeper pelvic tissues, and the focused applicator makes it easier to target the uterus, ovaries, and sacrum precisely instead of spreading a weaker, non‑contact dose over a large surface area. The Fringe wand brings light directly to the uterus and ovaries (within a few centimeters).
Q6: How often should women treat if they’re trying to mirror the IVF trial?
Both new trials used six sessions. A practical adaptation is to aim a similar level of Solasta treatments and Fringe Wand in that same window. For longer‑term ovarian and pelvic support, observational protocols and translational work commonly use several sessions over 3 cycles is usually the most effective approach.
Q7: Is more energy always better?
No. PBM follows a biphasic dose response: too little light has no effect, a moderate range helps, and excessive dosing can flatten or reverse benefits. Very high surface joules do not necessarily mean more benefit at the ovary or endometrium; what matters is staying within an effective window at depth and giving tissues time to respond.
Q8: Can PBM replace IVF or medication?
PBM should be seen as an adjunct to conventional fertility care, not a replacement. Women should always discuss any new therapy, including home laser use, with their fertility specialist - especially around embryo transfer, medication cycles, or early pregnancy.
Q9: Is it safe to use in early pregnancy?
Most clinical protocols focus PBM before conception or embryo transfer and often taper or stop once pregnancy is confirmed, mainly because human data on early pregnancy exposure are still limited it is generally advised to avoid putting the laser on the uterus during pregnancy.
Why I Offer Professional Laser Services and Encourage Home Use
Women often ask: “If you have a laser clinic, why would you encourage home use instead of more in‑person sessions?” The answer is about ethics, access, and matching the tool to the woman.
Access and geography. Multi‑session, cycle‑timed PBM is difficult if you live far from a clinic, lack childcare, or have limited time off. A home device allows more women to benefit from PBM’s uterine and ovarian effects regardless of postcode or schedule.
Cost and time. Clinical series and research on ovarian aging and uterine receptivity often involve weeks to months of repeated sessions. For some women, a focused clinic series before IVF makes sense; for others, especially those facing months of TTC, home PBM is more financially sustainable.
Consistency and timing. Studies that show benefits (RIF, ovarian aging, multi‑cycle fertility series) all rely on repetition at specific points in the cycle. A home protocol makes it easier to hit those critical windows than relying solely on clinic appointments.
Individualisation instead of one‑size‑fits‑all. Clinic‑based care with an expert is invaluable for complex, high‑risk, or unclear situations. Home‑based PBM can be enough for women with clear diagnoses, stable health, and good support and tailored protocols, especially when integrated with their existing medical team.
“With what we now know about red light therapy for the uterus and ovaries, it would be dishonest to suggest that weekly in‑person treatments are the only reasonable option when geography, cost, and the need for consistent, cycle‑timed sessions make an at‑home protocol a better fit for so many.”
Why You Don’t See Exact Protocols in My Blogs
PBM is powerful precisely because it is dose‑sensitive:
The same settings can be too little for one woman and too much for another, depending on skin, depth, diagnosis, and medications. Unfortunately there are some women who are ‘non-responders’ in a clinic or using my home laser we can tweak the protocol and use different wavelengths such as 1064 nm – known for deep penetration.
Canopy systems can’t be changed – it’s one size fits all.
Light has a therapeutic window, too little does nothing, and too much can flatten the benefits.
The right timing, intensity, and focus areas depend on your age, diagnosis, medications, and overall health picture.
Publishing generic ‘fertility laser protocols’ without that context would be misleading at best and counterproductive at worst.
How Protocols Are Accessed in My Work
If you choose to work with my devices:
You complete a detailed health and fertility intake, covering any diagnoses (e.g., RIF, DOR, PCOS, endometriosis), medication, cycle history, and current fertility plan.
I map your situation against the available evidence - uterine RIF trials, ovarian‑aging data, multi‑cycle fertility series - and then design a PBM plan that reflects those principles but is tailored, not copied from a paper.
I adjust or pause protocols if clinical situations change (e.g., new diagnosis, pregnancy, new medications).
If you decide to use my laser or other devices I work with, you don’t just receive a device; you receive a plan. That plan is anchored in published research on uterine receptivity, ovarian aging, and real‑world fertility case series - but it is written for your body, not for the internet.
Resources:
Low-Level Laser Therapy for Improvement of In Vitro Fertilization Outcomes in Women With Recurrent Implantation Failure – Clinical and Experimental Reproductive Medicine, 2024 (With a typical gynecologic probe spot of about 1 - 3 cm², that works out to an estimated 50 - 150 J/cm² at the skin per session, remember only a small fraction of this reaches the endometrium.
Effect of Intrauterine Laser Therapy on IVF Success on Infertile Patients: https://www.scirp.org/pdf/asm2024143_11990204.pdf
(Ohshiro/Kawano) - Personal Overview of the Application of LLLT in Severely Infertile Japanese Females (2012) (at around 60 mW/cm² for 20 minutes over the lower abdomen, giving roughly 72 J/cm² per session at the skin, repeated over multiple sessions and cycles). Folllicular and Ovarian function.
Animal Studies
Photobiomodulation ameliorates ovarian aging by alleviating oxidative stress and apoptosis in granulosa cells” (2024), 650 nm light at 6.7 mW/cm² with doses of 2–4 J/cm² to the ovaries and reported improvements in follicle counts, hormone profiles, angiogenesis, oxidative stress markers, and mitochondrial function in aging mice.